In vitro test reagents, including clinical diagnostic test kits and rapid diagnostic test kits, have a wide range of applications. These diagnostic tools can quickly and accurately test a variety of samples like blood, urine, saliva, sweat, serum, and other fluids for specific target molecules or gene expression. Clinical laboratories, hospitals, and physicians rely on rapid tests for diagnosis, prevention, treatment monitoring, and prognosis observation.
In addition, lateral flow assays are also used for food and environmental safety and veterinary medicine to identify chemicals such as diseases and toxins. Medical diagnostic kits, such as the COVID-19 Ag, are crucial tools for disease identification and control.

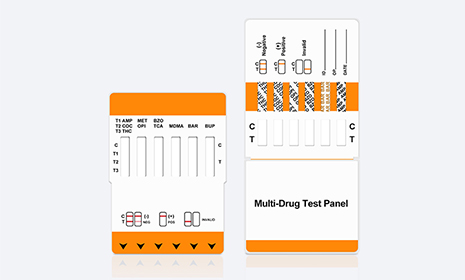
Human rapid diagnostic test (RDT) is a in vitro diagnostic rapid test that quickly and easily confirms whether a human sample reacts positive for the item to be tested. Classified as medical devices, RDT includes reagents, test kits, quality control products and other components used for in vitro detection. It can be used alone or in combination with instruments, devices or smart system.
The first category is for family self-examination of diseases, allowing people to independently complete some disease detection operations in their own homes according to the instructions. This can be used for self-diagnosis of various diseases, including infectious diseases, gastrointestinal diseases, and female hormone changes, as well as therapeutic effect monitoring for conditions like tumor marker chemoradiotherapy. The other kind is self-checking care family products, including health monitoring for blood lipid, blood glucose, and female genital tract health, as well as evaluations for bone health, nutritional status for vitamins and minerals, food safety for milk antibiotic residues, clenbuterol pork, and household environment, such as formaldehyde, allergens, and mites. These kits also allow assessment of health interventions and their effects on human health. Frenovo's home diagnostic kits and self-diagnosis kits offer a convenient and reliable way to monitor one's health at home.
Under the IVDR, the first step of compliance certification is to determine the classification of the product under the EU IVDR. Then what are the classification rules of Vitro Diagnostic Medical Devices (IVDR)?
The use of classification rules should be based on the intended use of the device.
If the device is to be used with other devices, the classification rules shall apply to each device separately.
Accessories in an in vitro diagnostic medical device should be separately classified from the device used in conjunction with it.
Software that drives a device or affects its use should be placed in the same category as the device.
Family self-examination is guided by the theory of general practice, and its basic functions are:
Health management companies that provide home self-examination services are aimed at the general public, but their direct customers may be health insurance companies. Home self-examination can avoid excessive medical care and reduce waste: Using the family as the "gateway" between the first doctor and the health care system can effectively reduce unnecessary tests and excessive treatment and avoid waste.
This way of physical examination can be carried out at home fully reflects the "people-oriented" humanized care, so that the physical examination becomes more warm and willing to accept. Health management companies that provide home self-testing services are also working as friends to fully negotiate and communicate with customers within the scope of medical principles so that individuals can understand and participate in the entire process of testing and decision-making. Many cases, such as pregnancy testing, testing whether children are drug addicts, testing whether they have AIDS, etc., have a certain degree of privacy, and this kind of home self-testing program is more popular among people.
Home self-examination is prevention-oriented medical care that can be targeted at all stages of health, illness and recovery, and can be at any time, anywhere, and always pay attention to the health of the individual.
Looking for top-quality in vitro diagnostic products? Look no further than Hangzhou Frenovo Biotech Co.,Ltd. - one of the leading diagnostic rapid test kit manufacturers in the industry. Trust Frenovo for all your diagnostic needs.
Lateral flow collodial gold tests called lateral flow immunochromatographic assays or rapid tests, are simple devices intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. These tests are widely used in medical diagnostics for home testing, point of care testing, or laboratory use. For instance, the home pregnancy test is an rapid test that detects a certain hormone. These tests are simple, economic and generally show results in around five to 30 minutes. Some usage increase the sensitivity of simple by employing additional dedicated equipment.
Rapid point-of-care testsor rule out COVID-19 infection in people with or without COVID-19 symptoms. They:
are portable, so they can be used wherever the patent is ( at the point of care );
are easy to perform, with a minimum amount of extra equipment or complicated preparaton steps;
are less expensive than standard laboratory tests;